
Electrooxidation of per- and polyfluoroalkyl substances in chloride-containing water on surface-fluorinated Ti4O7 anodes: Mitigation and elimination of chlorate and perchlorate formation. Mechanisms and pathways of PFAS degradation by advanced oxidation and reduction processes: A critical review. Mohamed Gar Alalm, Daria Camilla Boffito.Frontiers of Environmental Science & Engineering 2023, 17 Recent advances in electrochemical decontamination of perfluorinated compounds from water: a review. Fuqiang Liu, Shengtao Jiang, Shijie You, Yanbiao Liu.Environmental Science & Technology 2021, 55 Near-Quantitative Defluorination of Perfluorinated and Fluorotelomer Carboxylates and Sulfonates with Integrated Oxidation and Reduction. Bentel, Yaochun Yu, Changxu Ren, Jinyu Gao, Vivek Francis Pulikkal, Mei Sun, Yujie Men, Jinyong Liu. Application of Heterojunction Ni–Sb–SnO2 Anodes for Electrochemical Water Treatment. Yi Zhang, Yang Yang, Shasha Yang, Estefanny Quispe-Cardenas, Michael R.Electrochemical Oxidation of 6:2 Polyfluoroalkyl Phosphate Diester-Simulation of Transformation Pathways and Reaction Kinetics with Hydroxyl Radicals. Jonathan Zweigle, Boris Bugsel, Markus Schmitt, Christian Zwiener.Defluorination of Omega-Hydroperfluorocarboxylates (ω-HPFCAs): Distinct Reactivities from Perfluoro and Fluorotelomeric Carboxylates. Bentel, Yaochun Yu, Yujie Men, Jinyong Liu. Electrically Controlled Anion Exchange Based on a Polypyrrole/Carbon Cloth Composite for the Removal of Perfluorooctanoic Acid.

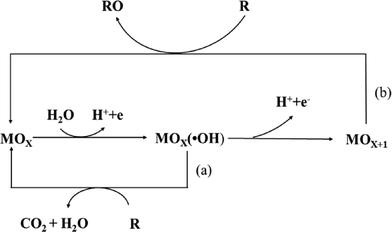
More comprehensive and rigorous evaluation of novel electrode materials, application of scalable proof-of-concept studies, and acknowledgment of all treatment outputs (not just the positive ones) are imperative. Nevertheless, the first step toward advancing from laboratory-scale to industrial-scale applications is recognizing both the strengths and limitations of electrochemical water treatment systems. In addition, the formation of organohalogen byproducts, chlorate and perchlorate, was seldom considered. Electrooxidation experiments conducted with high initial PFAS concentration and/or in high conductivity supporting electrolytes likely overestimate process performance. PFASs are surfactant molecules, which display significant concentration-dependence on adsorption, electrosorption, and dissociation. We have identified several shortcomings of the existing studies that are largely limited to small-scale laboratory batch systems and unrealistic synthetic solutions, which makes extrapolation of the obtained data to real-world applications difficult. In this review, we discuss the state-of-the-art on electrooxidation of PFASs in water, aiming at elucidating the impact of different operational and design parameters, as well as reported mechanisms of PFAS degradation at the anode surface. Electrochemical treatment systems have the unique ability to completely mineralize poly- and perfluoroalkyl substances (PFASs) through potential-driven electron transfer reactions.


 0 kommentar(er)
0 kommentar(er)
